The 5 Most Common Revelations Experienced in Process Analyzer System Training: A 50 Year Retrospective

The 5 Most Common Revelations Experienced in Process Analyzer System Training
Tony Waters, Sampling System Expert, Instructor
A process analyzer sampling system is one of the most challenging systems within your plant. Not only is it challenging to design, it’s also challenging to operate accurately. If you make one minor adjustment here, you may end up having to make a major one elsewhere. It’s no wonder Swagelok gets so many good questions, and attentive trainees, during our process analyzer sampling system (PASS) training courses. Over the last 50 years I have been teaching, I have observed students experiencing many epiphanies. Here are the top five “aha” moments from my process analyzer system trainees over the years:
"Time Delay is Often Longer than I Expected”
Most trainees do not consider the importance of addressing time delay in an analytical instrumentation system and are often amazed at how late some analyzer measurements are received. The industry standard is around one-minute for a response time—from pulling a sample to obtaining a reading. This short time frame provides near real-time readings of process conditions, allowing you to make immediate adjustments and minimize wasted product.
However, in some cases, the time to obtain a reading can be extensive—even when the analyzer is mounted just a few feet away from the process tap. These delays become an issue when they exceed the sampling system designer’s expectations. An inaccurate estimate or wrong assumption about time delay can result in inadequate process control.
The only way to reduce time delay is by adjusting your system design. We do a practical exercise in our process analyzer system training courses in which we calculate the time delay in a typical sampling system. In the exercise, our initial design has an enormous delay of more than five hours. But after making some quick system modifications, we bring that delay down to the one-minute industry standard. Trainees are amazed at this process and go back to their own facilities and implement.
"My Sample May Not be Representative of the Process Conditions”
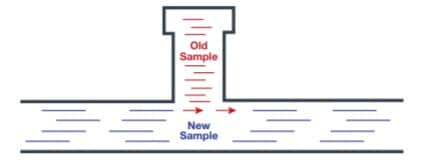
Time delay is such a critical issue to correct because it affects the “representativeness” of your sample reading. In other words, it reflects how representative the sample is of the fluid in the process line at the time you obtain your analyzer reading. For example, a process analyzer system with five-hour or more delay. If a negative reading occurs, the system operator will correct the quality issue and believe the problem is resolved. However, the operator isn’t likely aware that the reading was delayed more than five hours. During that time, plenty of inferior product went through the system and may already have been shipped to a customer.
Even when you take a proper sample and limit the time delay, it may still become unrepresentative due to your sampling system’s design. For example, deadlegs or dead spaces in the process analyzer system may trap old samples that can bleed into the new one, creating a mixed sample that isn’t true to real-time process conditions.
Your sample may also become contaminated due to leaks—not leaks from the sampling system itself, but leaks into the system from surrounding ambient air. For instance, oxygen can leak into a system containing 100 percent nitrogen at 100 psia because the partial pressure of oxygen outside the system is greater than its partial pressure inside the system. This kind of leak can be solved by increasing the partial pressure of the sampling system to avoid unintentional ambient air contamination.
“I Need to Pay More Attention to my Coalescers”
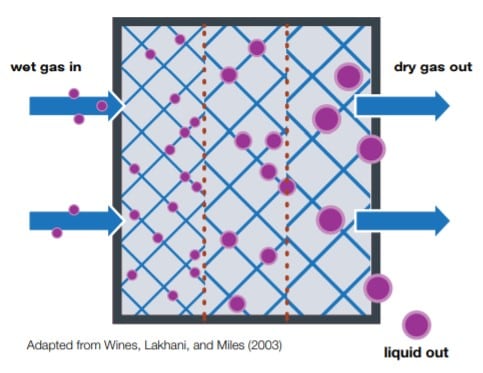
In sample conditioning training, most attendees think a coalescer is a device used to separate and remove liquids from a gas sample. While this is partly true, this belief is only accurate for liquids suspended in aerosol form. Aerosol is present in many gas samples because acceleration devices like cyclones or gravity separators are unable to separate liquid droplets. A coalescer installed in the sampling system allows the small droplets to come together and combine into large drops that separate more easily by gravity.
Trainees are surprised to learn two conditions will typically make a coalescer ineffective. First, free liquid (i.e., liquid that is not aerosol) will flow right through a coalescer with hardly any separation due to the massive size of the liquid droplets. Secondly, when the flow rate through a coalescer is too high, the fine aerosol droplets will get pushed past the coalescer elements and won’t be removed from the flow path. Both scenarios increase the potential of aerosol droplets reaching the analyzer and reducing the reliability of your readings—rendering the coalescer useless.
“Vaporizing a Liquid can be Problematic”
Many trainees think vaporizing a liquid sample is easy—but a lot can go wrong. The goal is to convert the liquid to a vapor state instantly by dropping the liquid’s pressure rapidly. However, instead of flashing the whole sample into a vapor, you could unintentionally create a fractionated sample through a combination of vaporization and evaporation. Once a sample fractionates, it is no longer suitable for analysis.
In such a scenario, lighter gas molecules evaporate first and move downstream to the analyzer, while heavier molecules remain behind in the liquid phase. As a result, the sample reaching the analyzer no longer accurately represents the product taken from the process line. By understanding what occurs during vaporization and learning more about managing vaporization in an analytical system, you can prevent this occurrence from happening in the future.
"Condensation can be Challenging—but Fixing it is Simple”
Condensation is perhaps the most common issue experienced with gas samples. Trainees are surprised to learn how quickly gases cool down (and how slowly liquids do). However, they’re also happy to learn that it’s easy to predict when condensation will occur and what temperature is required to stop it from happening.
Consider a system that reduces the pressure of a gas sample in a field station, which should be located as close to the tap as possible. Remember, almost all gases lose heat during a pressure drop (a phenomenon known as the Joule-Thomson effect). If your pressure drop is miniscule, you can likely use a simple pressure-reducing regulator without worry of producing condensation. However, a significant gas pressure drop will cause condensation due to the significant heat loss. This is even more likely when the gas is close to its dew point temperature.
Process analyzer sampling system design is a lifelong journey of discovery. There is always something new to figure out. After 50 years of experience with sampling systems, I’m still learning myself – and even achieve an “aha” moment of my own every now and then. Training is key to enhancing your skills and discovering what you still have left to learn. Even when you’re out in the field, you will likely experience some epiphanies of your own that may lead to more accurate and reliable process analyzer system readings for all.
About the Author
Tony Waters is an industry expert and consultant for Swagelok. With more than 50 years of experience with process analyzers and their sampling systems, he has worked in engineering and marketing roles for an analyzer manufacturer, an end user, and a systems integrator. He has founded three companies to provide specialized analyzer services to the process industries and is an expert in the application of process analyzers in refineries and chemical plants.

3 Rules for Analyzer Accuracy
Learn to avoid three common sampling system performance problems that can lead to analyzer inaccuracies, costing you time and money.

Sampling Systems: 8 Common Process Analyzer Accuracy Challenges
Sampling systems expert and veteran industry instructor Tony Waters offers plant managers and design engineers tested ways to identify and resolve 8 common challenges for process analyzer accuracy.

10 Tips to Improve Sampling Systems
Managing an analytical instrumentation operation is no small feat. Receiving consistent results can be a struggle for even the most seasoned engineers. Luckily, there are several simple tips your team can use to improve your sampling system.